Project 1
Project Leader: Tallie Z. Baram, MD, PhD
The overall Center integrates a multidisciplinary group of scientists to probe the developmental origins of vulnerability to mental illness, capitalizing on the established contribution of sensory signals during sensitive periods to brain circuit maturation.
The broad goal of Project 1 is to directly test the overarching hypothesis of the Center that fragmented and unpredictable early-life sensory signals (FRAG) contribute to subsequent impairments of pleasure / reward processes by disrupting the normal maturation of the underlying brain circuits.
The use of experimental systems contributes greatly to the overall Center, because human studies allow for correlations but not for interventional mechanistic experiments. In addition, Project 1 addresses the hypothesis at levels of analysis that are not possible in humans, including direct molecular and cellular approaches and direct access to brain tissue. Project 1 additionally employs overlapping imaging and epigenomic methods that synergize with human studies and enable inferences of common cross-species processes and mechanisms.
Example of the power of experimental systems:
New brain clearing methods couples with light-sheet microscopy enable direct visualization of circuits labeled fluorescently using viral-genetic tracing
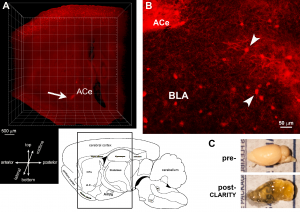
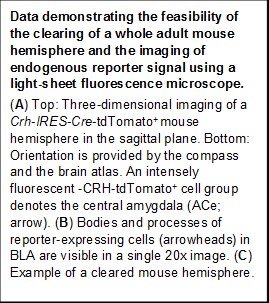
Testing the Center and project hypotheses will have major scientific and translational impact: These hypotheses address a novel type of early-life adversity, fragmented and unpredictable sensory signals, that disrupt normal maturation of brain circuits, culminating in outcomes including anhedonia and vulnerabilities to mental illness. Project 1 greatly advances the goals of the Center because it can establish causality of correlational observations made across species and provide mechanistic insight via intervention studies that target candidate mechanisms. These approaches further capitalize on species-unique opportunities including the access to brain tissue, genetic engineering and short life span, and employ cutting edge technologies.
Our approaches or conceptually and technically innovative
(a) The notion that patterns of sensory signals help sculpt brain circuits including those involved in pleasure/reward processing is novel. Whereas sensory signals drive the maturation of visual, auditory and sensory/motor circuits, their contribution to the pleasure / reward circuit has been largely unexplored.
(b) The use of intra-individual epigenomics is highly novel conceptually and technically. By focusing on the change in methylation patterns in the same individual (Pre vs Post) as a result of a short developmental exposure to defined patterns of sensory signals, the massive inter-individual variance is controlled for, enabling detection of epigenetic ‘scars’ and ‘kisses’. Upon implementation in infants, these might serve as predictive (bio)markers for mental vulnerability or resilience.
(c) Cross species application and translation of epigenomics and imaging methods are innovative.
(d) Technically, the use of gray-matter-DTI such as intra-NAc DTI is cutting edge.
(e) Genetic-viral tracing using CRE-directed viruses to delineate specifically cell-type specific projections from amygdala to NAc is state of the art, as is chemogenetic targeting of these fibers.
(f) Cell-specific knockdown of genes of interest to prevent emergent phenotypes is state of the art.
(g) Rodent resting-state fMRI using agents that do not suppress default networks is state of the art.
We capitalize on cross-species integration: identified by viral tracing and rodent DTI will synergize with findings in the child (Project 2) adolescent (Project 3) and adult (Project 4) to provide a deep understanding of the developmental trajectories of vulnerabilities to psychopathology.
We aim to address three key hypotheses:
1: That aberrant maturation of pleasure and reward circuits underlies FRAG-evoked anhedonia, using state-of-the-art structural (DTI) and functional (resting state fMRI) imaging.
2: That aberrant function of pleasure and reward circuits is a mechanism for FRAG-evoked anhedonia, using cutting-edge viral-genetic technologies.
3: That FRAG creates ‘epigenetic signatures’ and that provide predictive markers for emotional vulnerabilities in children.
Example of the power of experimental systems:
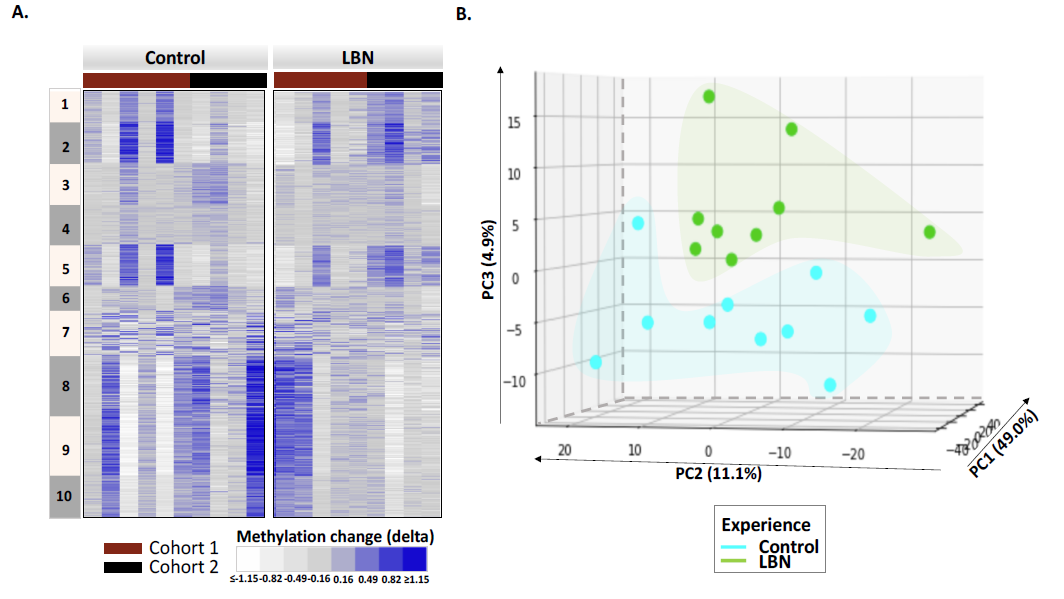
(A) Heatmap of intra-individual changes in methylation levels (delta methylation) between Postnatal day (P)10 and P2 (log2(P10/P2)) of 3,417 differentially methylated regions (DMRs). These DMRs are clustered using k-means. (B) Principal component analysis (PCA) of the DMRs enables distinguishing between individuals who have experienced FRAG/LBN (green) or routine (aqua) experiences during the intervening week between the collection of the pre- (P2) and post- (P10) samples. Thus, the changes in individual methylome provides an epigenetic scar of the adverse FRAG experience. Simple comparison of methylation levels at P10 across individuals does not.
Thus, Project 1 employs multiple experimental approaches and levels of analysis to test causality and provide mechanistic insights into the Center hypotheses, collaborating with all projects and cores.

